Microbial Transplantation Kit Market to Reach USD 891.8 Million by 2035, Driven by Clinical Adoption
Global microbial transplantation kit market set to nearly double by 2035, driven by AI diagnostics, oral therapies, and clinical adoption.
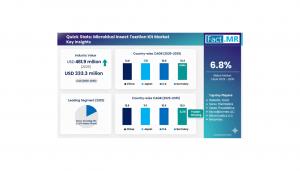
ROCKVILLE PIKE, MD, UNITED STATES, October 22, 2025 /EINPresswire.com/ -- The global microbial transplantation kit market is on a robust growth trajectory, projected to reach USD 891.8 million by 2035, up from USD 461.9 million in 2025, reflecting a compound annual growth rate (CAGR) of 6.8% during the forecast period. Increasing clinical acceptance, technological advancements, and government-backed biotech initiatives are transforming microbial transplantation kits from experimental tools into standardized therapeutic solutions for gastrointestinal and metabolic disorders.Microbial transplantation kits have become pivotal in the management of infectious diseases, particularly Clostridioides difficile infections and inflammatory bowel diseases. The adoption of standardized donor screening, encapsulation, and cryopreservation processes is accelerating across hospitals and biobanks in North America and Western Europe. Regulatory approvals, including the U.S. FDA clearance of therapies like Rebyota, combined with increasing clinician confidence and insurance reimbursement, are further solidifying the scalability of these solutions.
Market Drivers and Technological Innovation
The shift toward gut microbiome rehabilitation as a chronic disease management strategy has heightened demand for oral administration kits, minimizing reliance on invasive procedures. Automation in stool processing, cryopreservation, and sample viability management is significantly improving turnaround times in clinical laboratories. Simultaneously, the development of GMP-certified manufacturing centers in the U.S., France, and Japan underscores the transition from experimental therapy to regulated clinical practice.
Key drivers include AI-enhanced microbiome analytics, multi-omic donor profiling, and integration of predictive modeling for microbial compatibility. Hospitals and biobanks increasingly prefer modular kit systems that guarantee traceability, biosafety, and compliance with international standards. The deployment of machine learning for microbiome mapping, predictive donor-recipient matching, and cloud-based monitoring systems is revolutionizing the clinical application of microbial transplantation kits.
Regional Insights and Growth Hotspots
Asia Pacific is emerging as a critical growth hub, with China leading the charge through national health innovation programs and large-scale investments in GMP-certified facilities. India is also gaining traction, fueled by government initiatives to strengthen biopharmaceutical manufacturing and collaborative ecosystems between hospitals and microbiome startups.
In Europe, stringent regulatory controls and sustainability-focused healthcare policies are driving the adoption of certified microbial transplantation kits. Germany and France have been at the forefront of R&D in microbiome medicine, automation of donor processing, and clinical standardization. The U.K. has leveraged AI-based donor-matching algorithms and digital traceability systems, ensuring regulatory compliance and therapeutic consistency.
The United States remains a global leader, supported by strong clinical adoption, federal investment in biotechnology, and approval of live biotherapeutic products. AI-powered microbial recognition systems, advanced sequencing technologies, and specialized stool banks contribute to the reliability and availability of these kits. Canada is following suit, investing in bioinformatics and microbial production facilities to serve domestic and export markets.
Emerging regions, including Latin America and the Middle East & Africa, are entering early adoption stages, motivated by rising gastrointestinal disorders, medical tourism, and modernization of healthcare infrastructure in collaboration with global biotech companies.
Challenges and Restraining Factors
Despite these advancements, several challenges persist. Maintaining microbial viability throughout collection, processing, and storage requires specialized infrastructure, certified laboratories, and highly trained staff. Regulatory approvals remain resource-intensive, requiring biosafety validation, ethical donor certification, and pathogen screening per country-specific guidelines. The lack of harmonized international regulations complicates global commercialization, while clinical acceptance is hindered by low practitioner awareness, limited reimbursement, and inconsistent patient outcomes.
Price disparities, donor shortages, and logistical challenges in cold-chain transport further complicate large-scale adoption, particularly in developing regions where clinical microbiome infrastructure is nascent. Addressing these operational inefficiencies will be critical to sustaining market growth and ensuring broader clinical integration.
Product Segmentation and Clinical Trends
Donor screening kits dominate the market, representing 27.6% of the 2025 market share. These kits employ AI-enhanced diagnostics, genetic sequencing, and multiplex pathogen panels to ensure comprehensive evaluation of donor microbiota. Standardized donor screening has become a cornerstone of clinical practice, reinforcing physician confidence and therapeutic efficacy.
Non-invasive oral capsules, particularly enteric-coated and freeze-dried formulations, are rapidly gaining preference due to high patient compliance, ease of administration, and scalable clinical deployment. Innovations in micro-encapsulation, time-regulated delivery, and AI-guided design are enhancing precision and reproducibility, supporting broader outpatient and remote care frameworks.
Competitive Landscape
Leading market players include Rebiotix, Finch Therapeutics, Seres Therapeutics, Crestovo LLC, and MicroBiome Therapeutics LLC, alongside emerging innovators such as Enterome Bioscience, MaaT Pharma SA, OpenBiome, and Osel Inc. The competitive focus is on AI-driven donor screening, automated processing, cryopreservation systems, and digital traceability. Strategic alliances between biotech firms, diagnostic equipment vendors, and healthcare IT solution providers are shaping an ecosystem for end-to-end, application-specific microbial transplantation systems.
Recent developments, such as Finch Therapeutics’ successful U.S. patent litigation against Ferring Pharmaceuticals over fecal-transplant technology, highlight the importance of intellectual property and innovation leadership in this rapidly evolving market.
Request For DIscount :https://www.factmr.com/connectus/sample?flag=S&rep_id=11238
Buy Now This Report : https://www.factmr.com/checkout/11238
Outlook
The microbial transplantation kit market is poised for sustained growth, underpinned by technological innovation, regulatory approvals, and expanding clinical adoption. With AI-enhanced analytics, GMP-certified manufacturing, and non-invasive delivery solutions at the forefront, manufacturers and healthcare providers are presented with opportunities to redefine microbiome therapeutics. The decade ahead promises accelerated adoption, improved patient outcomes, and a strategic landscape for industry leaders to consolidate market presence and drive next-generation solutions in microbial transplantation.
Checkout Our Latest Published Related Publications:
Microbial Cell Banking Market https://www.factmr.com/report/1480/microbial-cell-banking-market
Antimicrobial Susceptibility Test Market https://www.factmr.com/report/4334/antimicrobial-susceptibility-test-market
Antimicrobial Regenerative Wound Matrix Market https://www.factmr.com/report/antimicrobial-regenerative-wound-matrix-market
Corneal Transplantation Surgical Instrument Package Market https://www.factmr.com/report/2665/corneal-transplantation-surgical-instrument-package-market
S. N. Jha
Fact.MR
+1 628-251-1583
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.